Process validation is actually a significant part of excellent assurance from the manufacturing market. It includes the collection and Examination of information to ensure that a process continually creates products which fulfill predetermined technical specs and excellent requirements.
This approach is based on gathered understanding and insights from comprehensive product or service and process studies. When developing the Command Strategy, it is best to choose into consideration the next:
Set up qualification (IQ): This stage includes verifying that tools is mounted according to the company’s technical specs and design and style demands. Operational qualification (OQ): Throughout this phase, the main focus is on testing the equipment and units to ensure they function according to the meant efficiency criteria.
The process validation lifecycle includes 3 stages: process structure, process qualification, and continued process verification. Let us take a closer examine Each individual of those stages:
By validating the process, firms might have self esteem from the regularity and dependability in their manufacturing methods, resulting in improved merchandise top quality, increased buyer fulfillment, and compliance with regulatory specifications.
Interaction abilities: Capacity to Obviously document validation actions and talk findings to cross-purposeful teams.
A great validation system ought to make certain that each period, each process, and each alter has actually been sufficiently evaluated just before implementation. Screening a sample of the final merchandise will not equate to conclusive proof that every one of the products and solutions inside a batch meet up with the specification.
Copy of various copies of these supplies, in full or in part, with the needs of business distribution is prohibited.
Developer's guideTechnical documentation for developers.Enable centerAssistance with onboarding and platform mastery.
The moment your plan is in position, it’s the perfect time to put it into action. Execution consists of functioning the process below managed problems when intently monitoring the crucial parameters. Imagine it being a Are living exam where you guarantee all the things operates throughout the outlined limits.
Possible validation includes validating a completely new manufacturing process in advance of its regime use. It involves detailed scheduling, execution, and documentation with the validation protocol.
Process validation is a scientific technique to make sure that a manufacturing process consistently produces a product of predetermined good quality. Within this detailed information, We are going to check out the necessity of process validation, The true secret actions concerned, regulatory specifications, and also successful implementation methods plus the potential issues which could occur.
Risk evaluation and mitigation: Incorporating chance assessment into your process validation assists discover probable concerns right before they develop into sizeable challenges. By evaluating attainable dangers linked to Each individual process move, you are able to carry out strategies to mitigate them, guaranteeing smoother functions.
Re-validation is definitely the process of repeating process validation read more in order that any adjustments made while in the process or devices, as per change Management treatments, do not negatively effects the process qualities and products high website quality. This is certainly vital due to the fact adjustments can probably alter the product’s efficacy, safety, and quality.
 Tony Danza Then & Now!
Tony Danza Then & Now!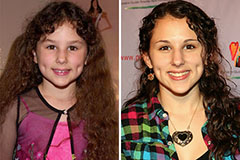 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Melissa Joan Hart Then & Now!
Melissa Joan Hart Then & Now! Seth Green Then & Now!
Seth Green Then & Now! Catherine Bach Then & Now!
Catherine Bach Then & Now!